
When there is doubt about the laboratory’s operations conforming to its own management system.When the evaluation of the nonconformance indicates a risk of it happening again.There are two primary reactive situations that trigger the need for a corrective action after a correction: Record it and monitor for any reoccurrence and change in risk level.

Never ignore an event, even if only a correction is necessary. The difficulty for many laboratories is deciding whether correction alone is sufficient. As the objective is to control the ongoing risk so that the same or a similar problem does not happen again, taking remedial action alone will require justifying the event as an isolated incident or why the current risk level is accepted. Corrective action, however, taking a risk-based approach, is not always necessary, and in some cases not possible. Corrections would be applied to every nonconformance. Not all nonconformances need corrective action.Ī correction addresses the short-term need, being a remedial reaction to control and correct the nonconformance. The following principles are a foundation for a suitable risk-based approach to corrective actions:
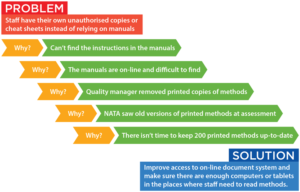
This means that laboratories should take a risk-based approach and should implement any action needed (in light of the risk). There is now no mandatory procedure required and laboratories can decide, based on evaluation, if there is a need for action to eliminate the cause(s) of the nonconformity. What has changed is the requirement for handling corrective actions. This is nothing new the same was required in ISO/IEC 17025:2005, the previous version. Where there is doubt about the compliance, or if there is a risk that the nonconforming work could recur, a decision must be made to follow the corrective action procedure. ISO/IEC 17025:2017 requires laboratories to follow a procedure to handle nonconformances.

When a laboratory’s results or activities do not conform to its own procedures or customer requirements, the undesired situation is classified as a nonconformance. What has changed in the new revision of ISO/IEC 17025?


 0 kommentar(er)
0 kommentar(er)
